
Epilepsy
Epilepsy is a neurological condition that makes people susceptible to seizures.
A seizure is a change in sensation (vision, hearing, touch, smell, taste), behaviour or abnormal movement involving a part of or entire body with or without losing awareness. It is brought about by a brief electrical disturbance in the brain. Seizures are sometimes called convulsions or fits. However, many seizures do not involve convulsions and can go unnoticed by bystanders.
Approximately 75% – 90% of individuals with TSC (Tuberous Sclerosis Complex) will have Epilepsy at some point. Epilepsy can start at any age, but many babies with TSC have a specific type of seizure called infantile spasms. Infantile spasms present with head bobbing, head and eye turning or whole body stiffening. They peak during the first 4-6 months of life but can occur anytime in the first two years.
Here is a glossary of some of the health care professionals who may be involved in managing epilepsy:
Neurologist – a doctor who specialises in brain and nerve related conditions
Epileptologist – a neurologist who specialises in treating epilepsy
Epilepsy specialist nurse – a senior nurse who may assist with epilepsy management, safety advice and education
Neurosurgeon – a doctor who performs surgery on brain and spine. An individual with TSC with medically uncontrolled epilepsy can be considered for epilepsy surgery
For more general epilepsy information, please visit Epilepsy Action Australia.
Why do seizures occur in TSC?
Seizures occur in TSC because the areas of abnormal brain development (tubers) cause abnormal electrical activity. It is not possible to predict whether an individual with TSC will have seizures, what type or how severe they will be.
Why are seizures different from one person to another?
The brain is made up of billions of nerve cells and has two halves, called cerebral hemispheres. Each hemisphere is made up of four areas (lobes) which are called the frontal, temporal, parietal and occipital lobes. Each lobe is divided by many clefts into smaller regions. Each region is responsible for specific functions like vision, hearing, touch, taste, smell, movement, judgement or memory. TSC brain lesions can involve any part of the brain, and the number, size and location of the lesions are variable in different people. Therefore, seizures are completely different from one person to another, depending on which brain area is affected. Moreover, brain changes in children mean their seizures can change as they grow older and their brain matures.
Signs, Symptoms and
Treatments

Signs and Symptoms
What type of seizures occur in TSC?
A distinct type of seizure that infants develop during the first 3-6 months of their life is called called infantile spasms. Otherwise, the seizures that occur in in TSC are focal seizures, which means they arise from a small abnormal region of the brain. These types of seizures were known as partial seizures in the past. A seizure may remain focal or spread to the nearby regions or the whole brain, causing generalised seizures.
Symptoms of focal seizures vary depending on the region of brain they start in and the regions they spread to. Some people can be completely conscious during the seizure and can talk about their feelings during the seizures. Other people can have loss of awareness or behavioural and emotional change, such as extreme fear. Other symptoms include movement of arms, legs, head or face in isolation or sequence, and falls. Seizures progressing to convulsions are called focal to bilateral tonic clonic seizures.
The types of seizures experienced may change throughout the life of someone with TSC and epilepsy.
This is due to brain maturation throughout life. The different changes that occur in the brain due to TSC are covered here. Impacts of TSC and epilepsy on learning, behaviour and mental health are covered here.
More on the different types of epileptic seizures
Though seizures in TSC start from a small abnormal area in the brain and therefore should cause focal seizures, some seizures rapidly or simultaneously involve multiple areas in the brain and cause bilateral tonic-clonic seizures.
Some of the more common types of seizures experienced by people with TSC are described below. For more information on seizure types, visit this information page from Epilepsy Action Australia.

- Motor seizure: Involuntary jerking of one or more parts of the body (focal motor seizures). Jerking may begin in a small area of the body and spread sequentially to arms, legs, or face on the same side. Jerking may also spread to the opposite side, causing a generalised convulsive seizure. Motor seizures may also present with bizarre movements such as paddling, jumping and or violent random movements of the arms and legs (hypermotor seizure). These often occur upon waking from sleep. They may be very short, occurring many times a day.
- Some people experience abnormal hearing, vision, taste, smell or tingling sensations. They may feel unexplained fear, sadness, joy or nausea. While an adult can describe these clearly, children who are preverbal or nonverbal may act strangely. Parents may notice that the child suddenly stops normal activities and become unresponsive. They may look pale, sweaty or have dilated pupils. They may return to normal after seconds or a few minutes.
- Seizures can start with or without an aura (an awareness of an impending seizure). The person may stare blankly, followed by repetitive movements such as chewing, lip smacking, swallowing, or grunting. The person will appear unaware of their surroundings; they may seem dazed and mumble; their actions may be clumsy and not focused. The person may pick at their clothing or objects; or even try to take clothes off. They may run, appear afraid, and may flail their arms and legs.
- Post-seizure confusion can last substantially longer than the seizure itself. The individual will have no memory of what happened during the seizure. At times, focal seizures can progress to bilateral tonic-clonic seizure, causing muscle stiffening and jerking.
Approximately one third of babies with TSC will develop infantile spasms. For some, this will be the first symptom that leads doctors to a diagnosis of TSC. Infantile spasms are clusters of brief stiffening movements that typically start between 3 and 18 months of age. They usually peak between 4-6 months of age, but can start anytime in the first two years of life.
The most frequent and common characteristic is a sudden forward movement of the neck (head nod) and the arms and shoulders jerk upwards and towards midline. They may look like they are doing a ‘sit up’. Extensor type spasms are less common and are characterised by sudden extension of the neck and the arms move away from the midline. In other cases, the spasms may be subtle, and only abrupt head nods occurring in clusters are noticed. In many cases, infantile spasms are a mix of characteristics.
Spasms tend to cluster, meaning they occur repeatedly 3-10 times in a row with a few seconds in between. At the end of a cluster the baby will often be very upset and will then be irritable. Spasms usually occur soon after the baby has woken up from sleep, or shortly after going to sleep.
Infantile spasms can difficult to diagnose and can be mistaken for normal body movements, colic/gastroesophageal reflux or startle reflex. As they can cause poor development, early correct diagnosis is important. Infantile spasm should be promptly treated.
Further information about infantile spasms can be found at TSC Alliance and Infantile Spasms Action Network.
Tonic seizures involve sudden lifting up and stiffening of arms, associated with stiffening of the neck and/or body. The legs can also show straightening and stiffening. In milder forms, these seizures can have subtle stiffening of the neck only.
During atonic seizures, the individual suddenly loses postural tone, resulting in a head nod or jaw drops (milder form), or falling to the ground (stronger form). Consciousness is usually impaired. The individual usually recovers after a few seconds to a minute.
Myoclonic seizures involve a sudden, involuntary, brief shock-like muscle contraction that usually involves both sides of the body, with synchronous jerks most often affecting the neck, shoulders, upper arms, body, and upper legs and may cause the person to spill what they were holding or fall off a chair.
Atypical absence seizures involve a prolonged stare or cessation of activity. They may begin and end gradually, usually last 5-30 seconds and are not generally provoked by hyperventilation. The person having the seizure may be partially responsive during episode. Eye-blinking or slight twitching movements of the lips may be seen. People with this type of seizure often have global cognitive impairment; therefore, it may be difficult to distinguish a seizure from the person’s usual behaviour. Electroencephalogram (EEG) is helpful to confirm the diagnosis of atypical absence seizures.
These are the most serious type of seizures and require prompt medical attention. Bilateral tonic-clonic seizure may occur with or without preceding aura/warning or complex partial seizure. Careful observation may reveal a seizure starting on one side with stiffening, jerking or head turn. There is often rapid progression to bilateral stiffening following by jerking. The seizure may last for many seconds to minutes.
A febrile seizure is a convulsion associated with a significant rise in body temperature. The incidence of febrile seizures is higher in TSC affected people (approximately 10%) than in the general population (5%). They are particularly common in children with TSC.
A person with TSC may suffer from both epileptic seizures and PNES. Unlike epileptic seizures, PNES present differently on each occasion and may last for minutes to hours. Patients are often at rest, lying or sitting with the eyes closed, or slowly drop to the floor and usually do not sustain any injuries. A detailed history of the symptoms and home videos of any episodes are helpful for a diagnosis. An absence of epileptic activity in EEG rules out an epileptic seizure.
It is reassuring to know that PNES does not occur as a result of abnormal electrical activity in the brain and therefore does not require antiseizure medications. A prompt referral to a psychiatrist and a psychologist is required for early intervention. Some antiseizure medications like Sodium Valproate and Lamotrigine are used in epileptic seizures and as mood stabilisers in PNES. Some people with PNES may find these medicines helpful.
Another way of describing the type of epilepsy a person has is as a syndrome. Diagnosis of an epilepsy syndrome is based on clinical and EEG characteristics. The two syndromes commonly diagnosed in people with TSC and epilepsy are:
1. Infantile Epilepsy Spasm Syndrome (IESS, previously known as West syndrome)
This is diagnosed on the basis of infantile spasms, the age at onset of spasms (under 12 months of age) and a typical EEG appearance – called hypsarrhythmia.
2. Lennox-Gastaut syndrome
This is diagnosed on the basis of multiple seizure types that occur in a person with TSC (particularly tonic, tonic-clonic and focal seizures), the age at onset of the different types of seizures (between 1 and 6 years of age) and a typical EEG appearance (slow spike and slow wave).
There have been many different studies looking to understand the link between epilepsy and other symptoms of TSC such as intellectual impairment, autism and mental health. Research has found that epilepsy starting early in life, infantile spasms and epilepsy that cannot be controlled may all increase the risk of intellectual disability and autism.
Although more research is needed to understand this better, the current research available does suggest that controlling seizures as early as possible may help to improve neurological outcomes. This is why many doctors will be aggressive in treating seizures, especially during the first 2 years of life.
Status epilepticus occurs when a seizure lasts too long, or when seizures occur close together without the person recovering between seizures.
*Status epielepticus is a medical emergency and requires immediate medical attention.*
1. Convulsive status epilepticus
This is defined as five or more minutes of continuous or repetitive tonic-clonic seizures without recovery of consciousness. Convulsive status epilepticus is a medical emergency and needs emergency treatment. If it is not treated urgently and successfully within 30 minutes, long term consequences with permanent brain damage or death may occur.
2. Focal status epilepticus with impaired awareness
This is defined as focal seizures of any type lasting for 10 minutes or more.
3. Absence or Non-convulsive status epilepticus
This is defined as continuous absence seizures lasting for 10-15 minutes. EEG is necessary for making a diagnosis of non-convulsive status epilepticus. Although it is not as severe as convulsive status epilepticus, it does require urgent treatment.
Anyone with epilepsy and TSC is at risk of status epilepticus. Possible triggers for this include an infection that results in a high temperature (fever), brain trauma, or missing doses of anticonvulsant medications.
There are different types of status epilepticus which may present with or without prominent motor symptoms and are defined by the duration of the seizures.
People living with seizures have a 1 in 1000 risk of SUDEP per year, although the risk is lower in children. Each individual with epilepsy will have a different risk depending on their seizure types, the frequency of seizures and other factors. You can discuss the risk of SUDEP with your neurologist.
The best way to reduce the risk of SUDEP is by improving seizure control.
Monitoring, Tests and Diagnosis
Diagnosis
Seizures are diagnosed based on clinical history. Repetition of similar neurological symptoms lasting for seconds to minutes are a clue for diagnosis.
Home videos using smart phones are valuable for doctors to confirm a diagnosis of clinical seizures and behavioural disturbance, and to decide what further tests needed to be done.
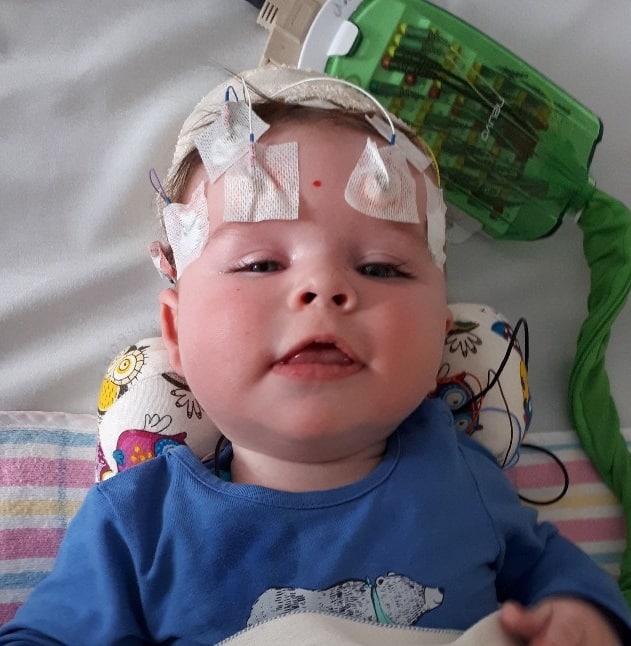
EEG
EEG (Electroencephalogram) is helpful for diagnosing electrographic seizures and figuring out which part of the brain the seizures are coming from, as well as performing pre-surgery screening in patients whose seizures are not controlled with medicine. EEG is also useful for diagnosing non-convulsive status epilepticus. It is a painless test that records the brain’s electrical activity, sometimes called brain waves.
Clinical trials suggest that babies with TSC who have not had any seizures should have regular EEG monitoring. This allows for early identification of epileptic EEG changes. This is becoming standard of practice around the world. Treatment at this early stage, prior to clinical onset of seizures, may seizures or infantile spasms. It may also make seizures easier to treat. This in turn may eventually reduce risk of neurodevelopmental delay and autism spectrum disorder. However, many more studies need to be done in this field.
For more information on EEG and the different types of EEGs, visit this page from Melbourne’s Royal Children’s Hospital.
Surveillance
Some individuals who have regular seizures (or their parents/carers) find it helpful to track seizures. Different methods can be used for this, such as paper and pen, using note taking app on the phone or computer. A mobile phone app called Seizure Tracker is one specific tool which can be used (developed by a TSC family in the USA).
Treatment
There are several different ways to treat epilepsy in individuals with TSC (Tuberous Sclerosis Complex).
In Australia Vigabatrin is the first line medication followed by oral steroid and Adrenocorticotropic Hormone (ACTH) for infantile spasms. You may hear concerns about the possible side effect Vigabatrin can have of narrowing a person’s field of vision. This is also called visual field loss. Screening can be done to monitor for visual field loss.
Read more about visual field loss in this whitepaper from the TSC Alliance
Any antiseizure medications used in other types of epilepsy can be tried for all other seizure types in people with TSC. Treatment may often be a case of trial and error and doctors rely on their own expertise, the experiences of their colleagues and published research to decide which treatment option to recommend in an individual with epilepsy related to TSC. Sometimes children and even some adults will need to take a combination of two or more anti-seizure medicines.
Everolimus (Afinitor) is an mTOR inhibitor medicine that has been proven to be effective and safe as an additional treatment for seizures for a person with TSC when other medications have not been able to stop seizures.
Everolimus works in a different way to other anti-seizure medications and it may take longer to see a reduction in seizures.
You can read more about these medicines on our mTOR inhibitors information page.
Clinical trials have shown long term safety and efficacy of cannabidiol (CBD) in patients with TSC. It is important to note that only the CBD form of cannabis has been trialled extensively for medicinal purposes (ie not THC). The safety and efficacy of other forms is unknown, and cannabis research is still in its infancy. Only one CBD product (Epidyolex) is Therapeutic Goods Administration (TGA) approved in Australia. It is only TGA and PBS approved for people 2 years or older with Lennox-Gastaut Syndrome and Dravet Syndrome. Other patients using the medication will pay out of pocket. The use of cannabidiol for other epilepsy syndromes can be cautiously considered by the neurology specialist on a case-by-case basis.
CBD is an alternate treatment to any other anti-seizure medication. Therefore, other anti-seizure medications are considered before CBD is trialed, as it is an expensive medication.
In TSC, cannabis-derived medicines are being researched to treat epilepsy. There are several research projects around Australia investigating the use of cannabis products to treat epilepsy. All of these studies are focusing on people for whom existing treatments have failed to control their seizures.
If you think that medicinal cannabis may be an option for you or someone in your family, you should speak with your neurologist. This is particularly important in a complex condition like TSC where it is also necessary to monitor possible interactions with other medications.
Elizabeth Thiele, neurologist and TSC Clinic director from Boston (USA) spoke about this area of research at TSA’s 2015 Australian TSC Conference. She concluded her conference session with this summary:
‘This is not a silver bullet for epilepsy in TSC. It is not the silver bullet for epilepsy in anything. But I think it is a very safe and well tolerated medication and I know it can be helpful for some people.’
If medication does not successfully control seizures in an individual with TSC, the ketogenic diet may be an option.
The ketogenic diet is a diet that is high in fat content and low in carbohydrates. Research suggest that it has been effective in treating epilepsy including individuals with TSC. This diet is usually used for children rather than adults due to its restrictiveness, although there have been a few individuals with TSC continuing the diet successfully into adulthood.
This diet is a medical regime and requires the help of an experienced dietician and neurologist. Neurologists work closely with dieticians in a multidisciplinary clinic to manage patients undertaking a ketogenic diet, or refer them to a dietician for continuing therapy.
The low glycemic index diet (LGIT) and modified Atkin’s diet are modifications of the ketogenic diet that are more palatable for some individuals. These epilepsy diets should never be tried without the help of a health professional as there are dangerous short and long-term side effects, so monitoring is required.
Epilepsy surgery can be an option when epilepsy persists despite multiple trials of antiseizure medications. Resection of the abnormal region of the brain may provide seizure freedom. However, a person with TSC often has many tubers in most parts of the brain. Therefore, identifying which tuber is causing the seizure needs extensive monitoring in a specialised surgical epilepsy centre. This involves staying in the hospital and monitoring brain activity with a video EEG, as well as multiple brain scans.
The pre-surgical evaluation will result in a recommendation on whether surgery is an option and if it is, what type of surgery is recommended. There may be a recommendation for two-stage surgery for selected patients. The first stage involves stereotactic EEG (SEEG) to confirm which tuber is causing the seizures. The ideal candidate for surgery is an individual with only one seizure type and only one area of their brain causing the seizure. It is also ideal if that area of the brain can be safely removed without causing loss of function. Only a few hospitals in Australia offer SEEG and epilepsy surgery. Whether or not SEEG is required, an experienced epilepsy team will work together with the family to perform the pre-surgical evaluation in surgical epilepsy centres.
A vagus nerve stimulator (VNS) improves seizures about half of patients with TSC. It can be offered to people who have drug resistant epilepsy, as well as people who are not suitable for resective epilepsy surgery. VNS implantation is a day procedure, taking approximately 60-90 minutes. It involves two small cuts: one in the upper chest to put a device like a pacemaker and another cut in lower part of the neck on the left side to wrap a lead around the vagus nerve. The VNS generator sends intermittent electrical signals to the brain via the vagus nerve. It is not fully understood how VNS works, but the theory is that the stimulation alters nerve pathways that lead to a seizure, as well as altering the neurotransmitter chemicals at the bottom of the brain (brainstem) to decrease overall brain excitability. A neurosurgeon performs the surgery and a neurologist with expertise in managing VNS adjusts the electrical signal so that it is at the right level for that person.
Read more information about VNS on this information page from Royal Children’s Hospital in Melbourne.
The 2021 guidelines for the surveillance and management of TSC recommend:
• Treat seizures other than infantile spasms similarly to that for other types of epilepsy. In individuals with TSC whose seizures are resistant to commonly used anti-seizure medications, the ketogenic/low-glycemic diet, vagus nerve stimulation, and epilepsy surgery can be of benefit.
• Treat infantile spasms with Vigabatrin as first-line therapy. Adrenocorticotropic hormone (ACTH) can be used as second-line therapy if Vigabatrin treatment is unsuccessful.
Last updated: 01 July 2025
Reviewed by: Dr Denise Chan, Paediatric Neurologist and Epileptologist, Sydney Children’s Hospital
Updated February 2023 following review by Dr Denise Chan, Paediatric Neurologist and Epileptologist, Sydney Children’s Hospital; Dr John Lawson, Neurologist and Rural, Regional and Remote Clinical Trial Enabling Program, NSW Ministry of Health; Prof. Lakshmi Nagarajan, Neurologist and Epileptologist, Perth Children’s Hospital; Dr Zebunnessa Rahman, Neurologist and Epileptologist, Westmead Hospital, Sydney
- Kwiatkowski D.J., Whittemore V.H. & Thiele E.A. (2010) Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim: Wiley-Blackwell
- Anti-epileptic drugs: how they treat seizures and their unwanted side-effects, Tuberous Sclerosis Association, viewed 9th April 2012
- Epilepsy in Adults with TSC, TSC Alliance, viewed 9th April 2012
- Epilepsy Surgery for Individuals with TSC, TSC Alliance, viewed 9th April 2012
- Infantile Spasms and TSC, TSC Alliance, viewed 9th April 2012
- Types of Seizures Affecting Individuals with TSC, TSC Alliance, viewed 29th April 2012
- Epilepsy Explained, Epilepsy Australia, viewed 29th April 2012 h
- Vagal Nerve Stimulation, The Royal Children’s Hospital Melbourne, viewed 9th April 2012, https://rch.org.au/cep/treatments/index.cfm?doc_id=3245
- French, J. A. et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet, doi:10.1016/s0140-6736(16)31419-2 (2016).
- Capal J.K et al., Influence of Seizures on Early Development in Tuberous Sclerosis Complex. Epilepsy Behav. 2017 May; 70(Pt A): 245–252.
Parts of this web page have been adapted with permission from copyrighted content developed by the TSC Alliance (tscalliance.org) and the Tuberous Sclerosis Association (tuberous-sclerosis.org).
