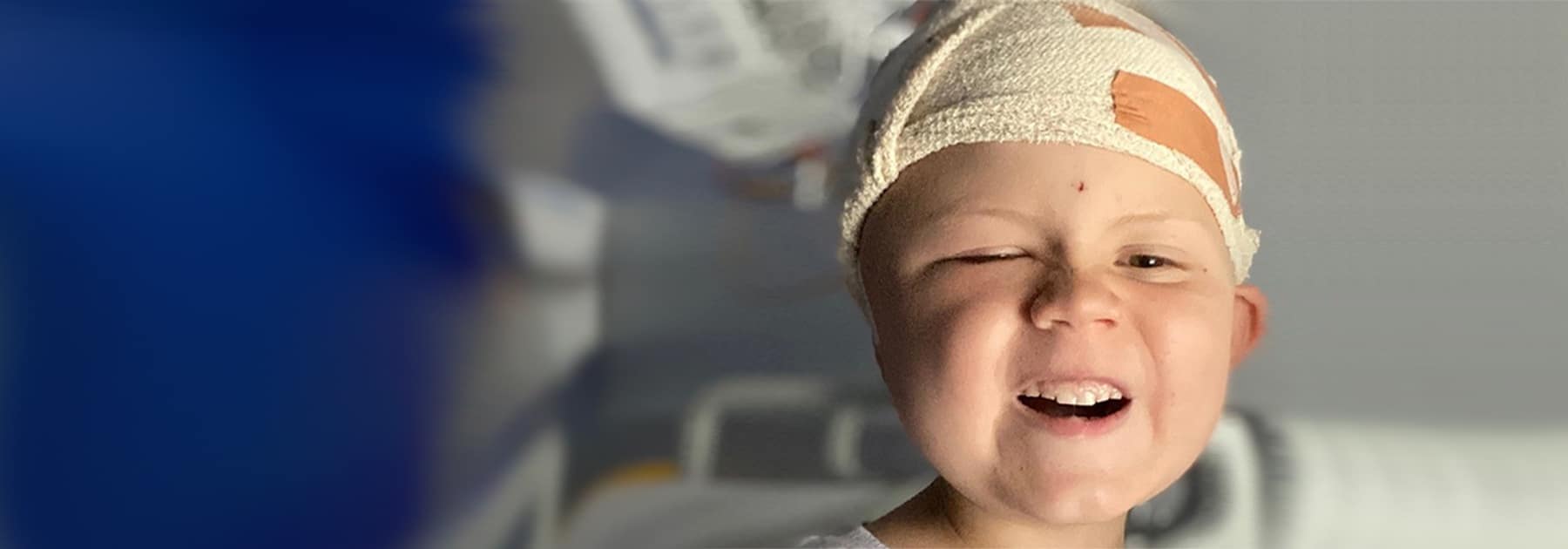
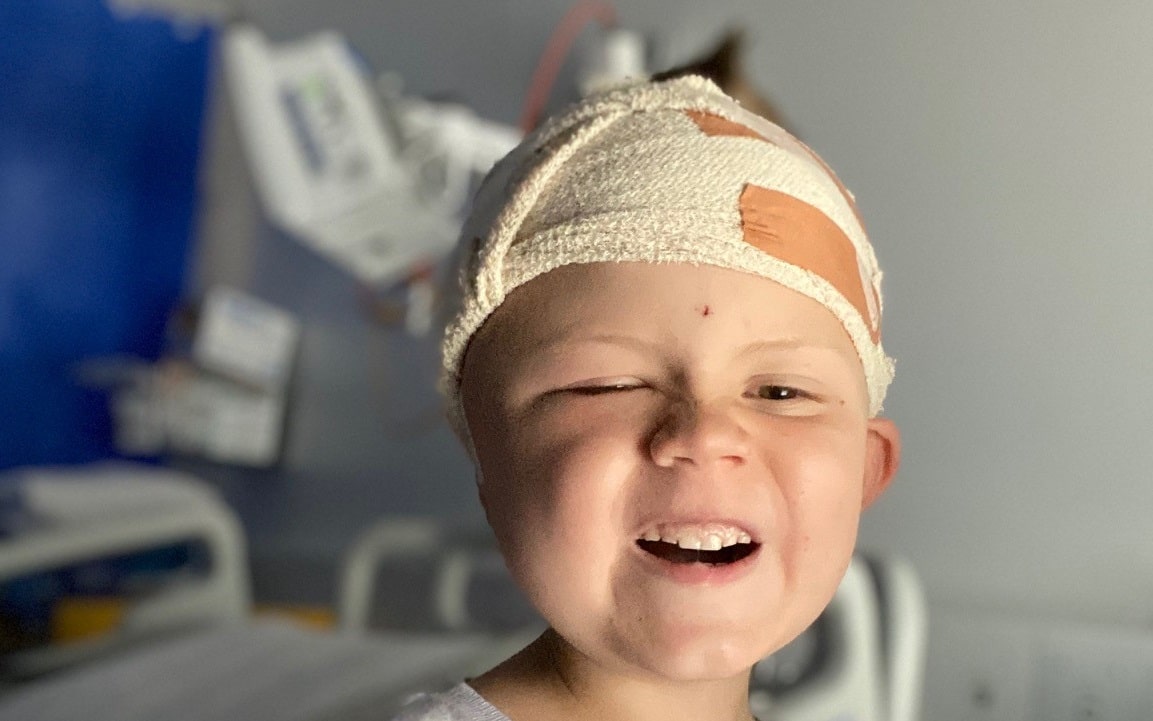
TSC (Tuberous Sclerosis Complex) is a rare genetic condition that causes tumours to grow in major organs of the body.
It affects more than 2,000 people in Australia and thousands more families and friends.
TSA is the only organisation in Australia supporting people living with TSC.
Learn
We provide access to accurate, balanced information about TSC.
Engage
We are here so that no-one has to face the challenges of TSC alone.
Support
We need your support to help change lives for people living with TSC.
Awareness is critical …
1 child each week
Every week in Australia another child is born with TSC. Two-thirds have no family history. Many children are not diagnosed until they experience symptoms such as infantile spasms or seizures.
1 million worldwide
Nearly 1 million people are estimated to have TSC. Life-long surveillance is essential. Help shine a light on TSC by getting involved in TSC Global Awareness Day held annually on 15 May.
Stories from our TSC Community
See all the Stories from our TSC Community